Marine Chemistry: The Role of Chemistry in the Ocean Environment 
Marine chemistry is the study of the chemical composition of seawater and the various chemical processes that occur within the marine environment. Oceans, which cover about 71% of the Earth’s surface, are a central component of the global climate system and support a wide range of chemical reactions that influence life on Earth. The chemistry of the ocean affects everything from nutrient cycling, biological productivity, and ocean circulation, to climate regulation and the global carbon cycle.
This article will explore the key components of marine chemistry, including the properties of seawater, the chemical composition of the ocean, important chemical processes in the ocean, the role of marine chemistry in climate regulation, and how human activities impact marine ecosystems through chemical changes in the ocean. By understanding the chemistry of the oceans, we can better appreciate their essential role in the Earth’s system, and how the health of the oceans is critical to the future of life on Earth.
- Properties of Seawater
Seawater is a complex mixture that is primarily composed of water (H₂O) and dissolved substances, such as salts, gases, and organic compounds. The chemical properties of seawater differ significantly from freshwater due to the presence of dissolved salts, especially sodium chloride (NaCl), as well as other minerals and gases.
1.1 Salinity
One of the most important characteristics of seawater is its salinity, which refers to the concentration of dissolved salts, mainly sodium chloride, in the water. The average salinity of seawater is about 35 grams per liter, but this can vary depending on location, depth, and environmental conditions.
- Variation in Salinity: Salinity levels are not uniform across the oceans. For instance, regions near the equator, where evaporation rates are high, tend to have higher salinities. On the other hand, polar regions or areas where there is significant river runoff, such as estuaries, tend to have lower salinities.
- Effects on Marine Life: Salinity affects the osmotic balanceof marine organisms, influencing how they regulate the intake of water and the excretion of waste products. For marine organisms, maintaining homeostasis (a stable internal environment) in water of varying salinity is a critical physiological challenge.
1.2 pH of Seawater
The pH of seawater typically ranges from 7.5 to 8.4, making it slightly alkaline. The pH of the ocean is primarily controlled by the balance between carbon dioxide (CO₂) in the atmosphere and the ocean’s ability to absorb it. When CO₂ dissolves in seawater, it reacts to form carbonic acid (H₂CO₃), which then dissociates to produce hydrogen ions (H⁺) and bicarbonate ions (HCO₃⁻).
- Ocean Acidification: Increasing levels of CO₂ in the atmosphere are leading to higher concentrations of dissolved CO₂ in the oceans, a phenomenon known as ocean acidification. This reduction in pH can have profound effects on marine life, particularly organisms that rely on calcium carbonate (such as corals and shellfish) to form their shells and skeletons.
1.3 Temperature and Density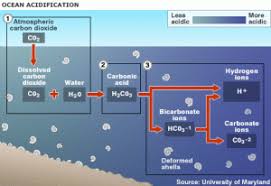
The temperature of seawater is another critical factor influencing chemical reactions in the ocean. Ocean temperature varies with latitude, with warmer waters found near the equator and colder waters at the poles. Temperature influences the solubility of gases, the rate of chemical reactions, and the distribution of marine life.
- Thermocline: The ocean is stratified into layers based on temperature, with the upper mixed layer being warmer and the deeper layers being colder. The boundary between these layers is called the thermocline, and it can affect the distribution of nutrients and gases in the ocean.
- Density: The density of seawater is determined by temperature and salinity. Cold water is denser than warm water, and seawater with higher salinity is denser than water with lower salinity. The difference in density drives the ocean circulationand is essential for nutrient mixing and the vertical movement of water.
- Chemical Composition of Seawater
Seawater is made up of numerous dissolved ions and compounds. The most abundant of these are sodium (Na⁺), chloride (Cl⁻), sulfate (SO₄²⁻), magnesium (Mg²⁺), and calcium (Ca²⁺), which together account for the vast majority of dissolved salts in seawater. Other important ions include potassium (K⁺), bicarbonate (HCO₃⁻), and various trace elements like iron (Fe), zinc (Zn), and copper (Cu), which play crucial roles in biological processes.
2.1 Major Constituents of Seawater
The major ions in seawater, listed in order of their relative abundance, include:
- Chloride (Cl⁻): The most abundant ion in seawater, accounting for about 55% of the dissolved ions. Chloride is a key component of the ocean’s salinity.
- Sodium (Na⁺): The second most abundant ion, making up about 30% of the dissolved ions.
- Sulfate (SO₄²⁻): Makes up about 7.7% of the dissolved ions. It is important in ocean chemistry and contributes to the formation of marine minerals such as gypsum and barite.
- Magnesium (Mg²⁺): About 3.7% of the dissolved ions, magnesium plays an important role in the formation of marine minerals and biological processes.
- Calcium (Ca²⁺): Around 1.2% of dissolved ions, calcium is crucial for the formation of calcium carbonate (CaCO₃) shells in marine organisms.
These ions are constantly involved in chemical reactions in the ocean, influencing everything from the formation of minerals to the availability of nutrients for marine life.
2.2 Trace Elements
In addition to the major ions, seawater contains trace elements such as iron (Fe), copper (Cu), manganese (Mn), and iodine (I). These elements are often present in very low concentrations, but they are essential for the biological processes of marine organisms.
- Iron (Fe): A key nutrient for phytoplankton, iron is often a limiting factor for primary production in the open ocean. Iron availability is influenced by inputs from dust, rivers, and hydrothermal vents.
- Copper (Cu)and Zinc (Zn): Both are important cofactors in enzyme systems involved in respiration and photosynthesis in marine organisms.
- Chemical Processes in the Ocean
Several critical chemical processes occur in the ocean, contributing to its dynamic nature and influencing the chemical composition of seawater. These processes are integral to the functioning of the marine ecosystem and the global biogeochemical cycles.
3.1 The Carbon Cycle
The ocean is a major carbon sink, absorbing large amounts of atmospheric CO₂. The chemical reactions in the ocean that involve carbon are central to regulating the Earth’s climate.
- Carbon Dioxide Exchange: Carbon dioxide dissolves in seawater, where it forms carbonic acid. This acid dissociates to produce bicarbonate and carbonate ions (HCO₃⁻ and CO₃²⁻). The balance between these forms of carbon is affected by ocean temperature, pH, and the concentration of dissolved CO₂.
- Biological Carbon Pump: Phytoplankton in the ocean take up CO₂ during photosynthesis, and when they die or are consumed by other organisms, carbon is transferred to deeper layers of the ocean. This process is known as the biological carbon pump and plays a vital role in the long-term storage of carbon.
- Ocean Acidification: The increasing levels of CO₂ in the atmosphere are leading to more CO₂ being absorbed by the ocean, resulting in ocean acidification. This lowers the pH of seawater and can have significant impacts on marine organisms that rely on calcium carbonate to form shells and skeletons.
3.2 Nitrogen and Phosphorus Cycles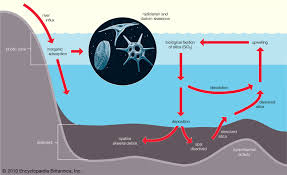
Both nitrogen and phosphorus are essential nutrients for marine life, supporting the growth of phytoplankton and other organisms. These elements cycle through the ocean in complex ways.
- Nitrogen: In the ocean, nitrogen is primarily found in the form of nitrate (NO₃⁻), nitrite (NO₂⁻), and ammonium (NH₄⁺). Nitrogen is a limiting nutrient in many parts of the ocean, and processes such as nitrogen fixation, nitrification, and denitrificationregulate its availability.
- Phosphorus: Phosphorus primarily exists as phosphate (PO₄³⁻) in the ocean. It is essential for DNA, RNA, and ATP production in organisms. Phosphorus is often a limiting nutrient in freshwater but can be abundant in the ocean.
3.3 Oxygen and the Biological Pump
Oxygen is critical for the survival of aerobic organisms, and its distribution in the ocean is influenced by biological processes.
- Oxygen Minimum Zones: In certain areas of the ocean, such as deep waters and areas with high biological activity, oxygen levels can be extremely low, forming oxygen minimum zones. These zones are typically located at depths of 200 to 1,000 meters and are influenced by the decomposition of organic matter and the lack of mixing in certain regions.
- Human Impact on Marine Chemistry
Human activities have significantly altered the chemical composition of the ocean. These impacts are often referred to as anthropogenic stressors and include:
- Ocean Acidification: As mentioned earlier, the burning of fossil fuels has led to higher concentrations of CO₂ in the atmosphere, much of which is absorbed by the oceans. This acidification can harm marine life, particularly organisms that rely on calcium carbonate to form shells, such as corals and shellfish.
- Pollution: Various forms of pollution, including plastic debris, heavy metals, and chemicals like pesticides, can disrupt marine chemical processes. Pollutants can alter nutrient cycles, disrupt marine food webs, and contribute to eutrophication, where excess nutrients cause oxygen depletion and dead zones.
- Nutrient Runoff: Excessive nitrogen and phosphorus from agricultural runoff and wastewater can lead to algal blooms, which deplete oxygen levels and harm marine ecosystems.
- Conclusion: The Importance of Marine Chemistry

Marine chemistry is central to understanding the ocean’s role in global processes like climate regulation, the carbon cycle, and marine productivity. The oceans are dynamic, with complex chemical processes that support life on Earth. However, these processes are being increasingly impacted by human activities, such as climate change, pollution, and overfishing. Understanding the chemistry of the ocean is not only essential for preserving marine ecosystems but also for ensuring the long-term sustainability of the ocean economy. Protecting and conserving the oceans is critical to maintaining the chemical balance that sustains life on Earth and regulating the planet’s climate.